FDA Issues New Mammography Guidelines for Women With Dense Breasts
4.7 (509) · $ 25.99 · In stock
The FDA on March 9 updated its mammography guidelines to require mammography facilities to notify patients about the density of their breasts.
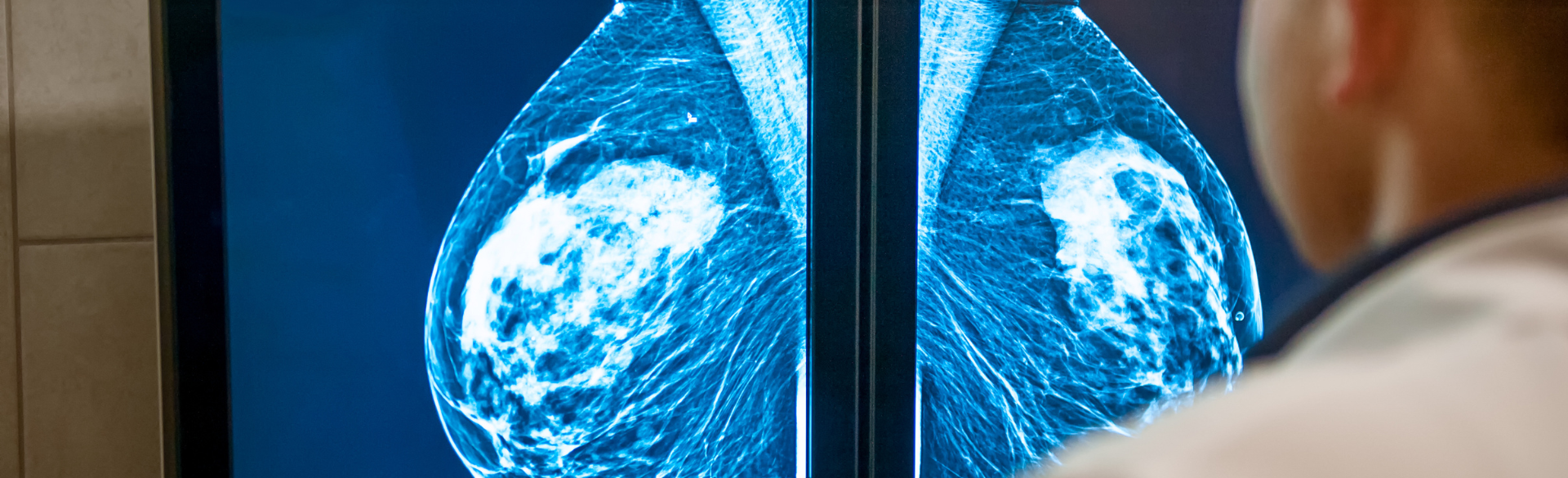
Breast density notification laws blanket 90% of U.S. women, yet still no national reporting standard is at hand. Why is that?
Breast Cancer
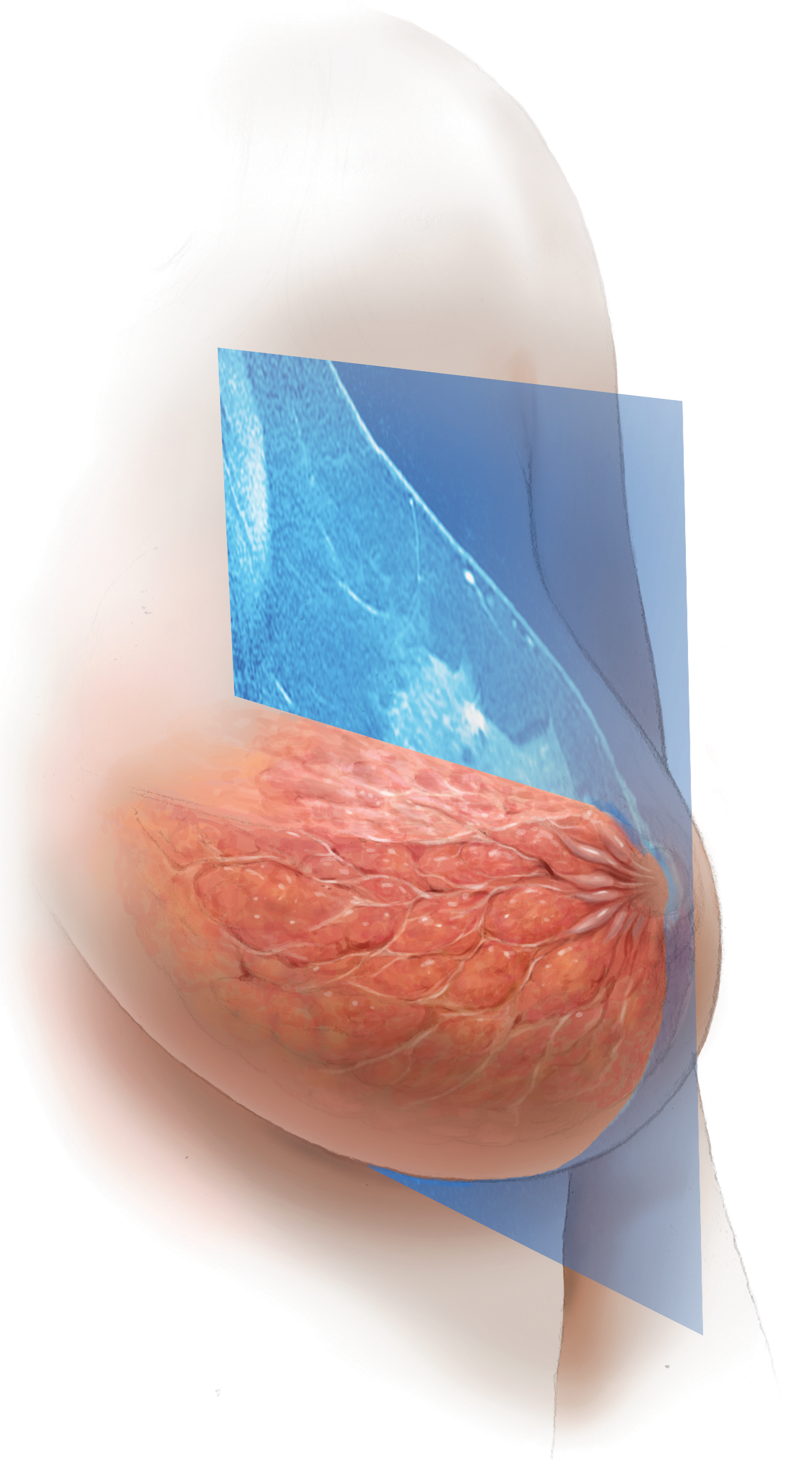
Breast density and optimal screening for breast cancer

FDA Issues New Mammography Guidelines for Women With Dense Breasts - Cancer Health

Your Healthy Family: FDA issues new regulations on dense breast tissue
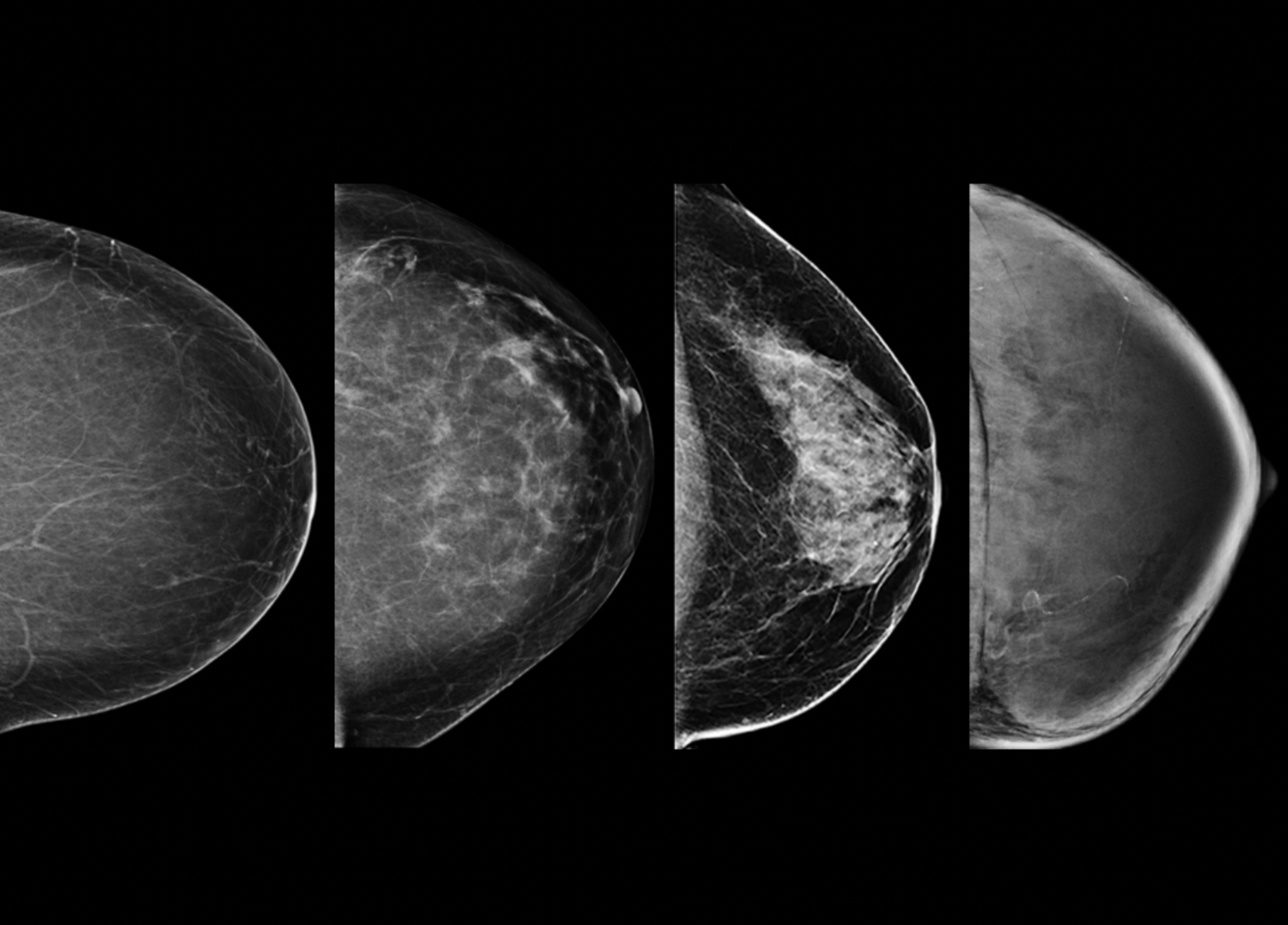
Detecting breast cancer in dense breasts
Mammography Quality Standards Act and Program

University of Colorado Cancer Center on LinkedIn: What to Know
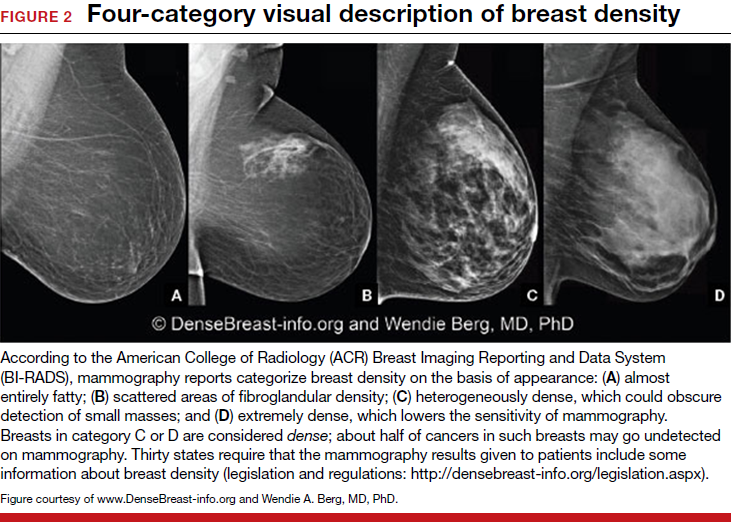
Breast density and optimal screening for breast cancer

Medical practitioners will have to notify patients about breast density in mammograms under new FDA regulations - CBS News

Breast Imaging Archives - Dr Deanna Attai
Breast cancer screening: FDA proposes mammogram changes

FDA Announces New Mammogram Standards for People With Dense Breasts